The Woolly Mammoth's Evolutionary History Over a Million Years Was a Complex Web






International space agencies stressed the importance of international partnerships with the United States and each other at Space Symposium April 9, as geopolitical shifts and an escalating trade war challenge the space industry.
The post Space leaders emphasize collaboration amid geopolitical challenges appeared first on SpaceNews.

COLORADO SPRINGS — The space industry must speed up adoption of artificial intelligence or risk falling behind, government and industry experts warned March 27. During a symposium organized by the […]
The post ‘Everyone is doing AI’: Space sector urged to catch up appeared first on SpaceNews.

Officials insist that U.S. and allies are keeping space cooperation on track
The post Space alliances hold strong despite broader geopolitical tensions appeared first on SpaceNews.

Plano, Texas – Apogee Semiconductor, a leading provider of advanced technologies for space and extreme environments, today announced its collaboration with Arrow Electronics, a global distributor of electronic components and […]
The post Apogee Semiconductor Teams with Arrow Electronics to Expand Distribution of Space-Grade Technologies appeared first on SpaceNews.

Our guest today is Kari Bingen, senior fellow at the Defense and Security Department at the Center for Strategic and International Studies. She sits down with Chief Content and Strategy Officer Mike Gruss, to break down the latest news and insights from the Symposium focusing on defense.
The post Signals from Space Symposium: A trillion-dollar defense budget appeared first on SpaceNews.



Here’s your SpaceNews show daily for Wednesday. Inside: a one-on-one with NASA acting administrator Janet Petro, a sharp commentary on accelerating space acquisition, and in-depth coverage of U.S. Space Command’s […]
The post SpaceNews’ full Wednesday show daily from Space Symposium — now live appeared first on SpaceNews.

NASA Administrator nominee Jared Isaacman said that the agency should simultaneously pursue both human missions to the moon and Mars.
The post Isaacman says NASA should pursue human moon and Mars programs simultaneously appeared first on SpaceNews.

Hyten: "You look at our on-orbit capabilities, they're basically the same as they were 10 years ago"
The post Defense experts warn procurement bottlenecks risk U.S. space edge appeared first on SpaceNews.

Saltzman calls space "the ultimate team sport" as U.S. seeks to strengthen alliances
The post Space Force to roll out strategy for international collaboration appeared first on SpaceNews.

COLORADO SPRINGS – The space sector is likely to benefit from increased defense spending by NATO allies, European military leaders said at the Space Symposium. At the NATO Summit in […]
The post Surge in NATO spending to benefit space sector appeared first on SpaceNews.

A team of researchers in Switzerland, Germany and the US has observed clear evidence of quantum mechanical interference behaviour in collisions between a methane molecule and a gold surface. As well as extending the boundaries of quantum effects further into the classical world, the team say the work has implications for surface chemistry, which is important for many industrial processes.
The effects of interference in light are generally easy to observe. Whenever a beam of light passes through closely-spaced slits or bounces off an etched grating, an alternating pattern of bright and dark intensity modulations appears, corresponding to locations of constructive and destructive interference, respectively. This was the outcome of Thomas Young’s original double-slit experiment, which was carried out in the 1800s and showed that light behaves like a wave.
For molecules and other massive objects, observing interference is trickier. Though quantum mechanics decrees that these also interfere when they scatter off surfaces, and a 1920s version of Young’s double-slit experiment showed that this was true for electrons, the larger the objects are, the more difficult it is to observe interference effects. Indeed, the disappearance of such effects is a sign that the object’s wavefunction has “decohered” – that is, the object has stopped behaving like a wave and started obeying the laws of classical physics.
In the new work, researchers led by Rainer Beck of the EPFL developed a way to observe interference in complex polyatomic molecules. They did this by using an infrared laser to push methane (CH4) molecules into specific rovibrational states before scattering the molecules off an atomically smooth and chemically inert Au(111) surface. They then detected the molecules’ final states using a second laser and an instrument called a bolometer that measures the tiny temperature change as molecules absorb the laser’s energy.
Using this technique, Beck and colleagues identified a pattern in the quantum states of the methane molecules after they collided with the surface. When two states had different symmetries, the quantum mechanical amplitudes for the different pathways taken during the transition between them cancelled out. In states with the same symmetry, however, the pathways reinforced each other, leading to an intense, clearly visible signal.
The researchers say that this effect is similar to the destructive and constructive interference of the double-slit experiment, but not quite the same. The difference is that interference in the double-slit experiment stems from diffraction, whereas the phenomenon Beck and colleagues observed relates to the rotational and vibrational states of the methane molecules.
The researchers had seen hints of such behaviour in experiments a few years ago, when they scattered methane from a nickel surface. “We saw that some rotational quantum states were somewhat weakly populated by the collisions while other states that were superficially very similar (that is, with the same energy and same angular momentum) were more strongly populated,” explains Christopher Reilly, a postdoctoral researcher at EPFL and the lead author of a paper in Science on the work. “When we moved on to collisions with a gold surface, we discovered that these population imbalances were now very pronounced.”
This discovery spurred them to find an explanation. “We concluded that we might be observing a conservation of the reflection parity of the methane molecule’s wavefunction,” Reilly says. “We then set out to test it for molecules prepared in vibrationally excited states and our results confirmed our hypothesis spectacularly.”
Because the team’s technique for detecting quantum states relies on spectroscopy, Reilly says the “intimidating complexity” of the spectrum of quantum states in a medium-sized molecule like methane was a challenge. “While our narrow-bandwidth lasers allowed us to probe the population in individual quantum states, we still needed to know exactly which wavelength we need to tune the laser wavelength to in order to address a given state,” he explains.
This, in turn, meant knowing the molecule’s energy levels very precisely, as they were trying to compare populations of states with only marginally different energies. “It is only just in the last couple years that modelling of methane’s spectrum has become accurate enough to permit a reliable assignment of the quantum states involved in a given infrared transition,” Reilly says, adding that the HITEMP project of the HITRAN spectroscopic database was a big help.
According to Reilly, the team’s results show that classical models cannot fully capture molecule-surface dynamics. “This has implications for our general understanding of chemistry at surfaces, which is where in fact the majority of chemistry relevant to industry (think catalysts) and technology (think semiconductors) occurs,” he says. “The first step of any surface reaction is the adsorption of the reactants onto the surface and this step often requires the overcoming of some energetic barrier. Whether an incoming molecule will adsorb depends not only on the molecule’s total energy but on whether this energy can be effectively channelled into overcoming the barrier.
“Our scattering experiments directly probe these dynamics and show that, to really understand the different fundamental steps of surface chemistry, quantum mechanics is needed,” he tells Physics World.
The post Quantum interference observed in collisions between methane molecules and gold surface appeared first on Physics World.

When Donald Trump returned to the White House in January, many in the space industry assumed there would be immediate changes at NASA, particularly in its human spaceflight programs. Elon […]
The post The continued momentum of Artemis appeared first on SpaceNews.

China has unveiled a long-term planetary exploration roadmap for planetary habitability and the search for extraterrestrial life. Released by the Deep Space Exploration Laboratory (DSEL), the roadmap outlines a series […]
The post China to seek out life in the solar system as NASA faces cuts, commercial players expand ambitions appeared first on SpaceNews.

Electrical engineer Ed Tate was skeptical of proposals for space-based solar power when he initially heard about the concept seven years ago. “My first reaction was, ‘That really sounds like […]
The post Startups are preparing for the launch of space-based solar power appeared first on SpaceNews.
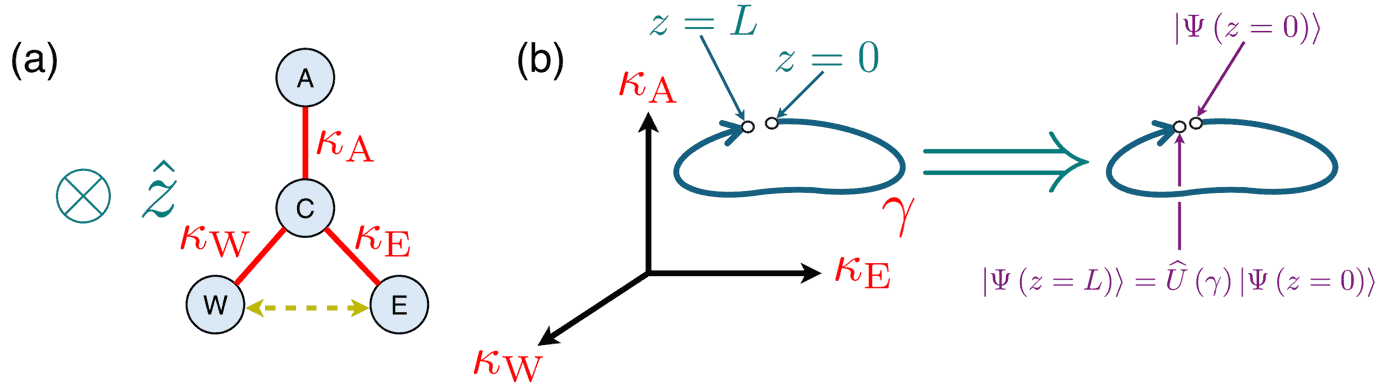
Physicists at the Georgia Institute of Technology, US have introduced a novel way to generate entanglement between photons – an essential step in building scalable quantum computers that use photons as quantum bits (qubits). Their research, published in Physical Review Letters, leverages a mathematical concept called non-Abelian quantum holonomy to entangle photons in a deterministic way without relying on strong nonlinear interactions or irrevocably probabilistic quantum measurements.
Entanglement is fundamental to quantum information science, distinguishing quantum mechanics from classical theories and serving as a pivotal resource for quantum technologies. Existing methods for entangling photons often suffer from inefficiencies, however, requiring additional particles such as atoms or quantum dots and additional steps such as post-selection that eliminate all outcomes of a quantum measurement in which a desired event does not occur.
While post-selection is a common strategy for entangling non-interacting quantum particles, protocols for entangled state preparation that use post-selection are non-deterministic. This is because they rely upon making measurements, and the result of obtaining a certain state of the system after a measurement is associated with a probability, making it inevitably non-deterministic.
The new approach provides a direct and deterministic alternative. In it, the entangled photons occupy distinguishable spatial modes of optical waveguides, making entanglement more practical for real-world applications. To develop it, Georgia Tech’s Aniruddha Bhattacharya and Chandra Raman took inspiration from a 2023 experiment by physicists at Universität Rostock, Germany, that involved coupled photonic waveguides on a fused silica chip. Both works exploit a property known as non-Abelian holonomy, which is essentially a geometric effect that occurs when a quantum system evolves along a closed path in parameter space (more precisely, it is a matrix-valued generalization of a pure geometric phase).
In Bhattacharya and Raman’s approach, photons evolve in a waveguide system where their quantum states undergo a controlled transformation that leads to entanglement. The pair derive an analytical expression for the holonomic transformation matrix, showing that the entangling operation corresponds to a unitary rotation within an effective pseudo-angular momentum space. Because this process is fully unitary, it does not require measurement or external interventions, making it inherently robust.
A classic example of photon entanglement is the Hong–Ou–Mandel (HOM) effect, where two identical photons interfere at a beam splitter, leading to quantum correlations between them. The new method extends such interference effects beyond two photons, allowing deterministic entanglement of multiple photons and even higher-dimensional quantum states known as qudits (d-level systems) instead of qubits (two-level systems). This could significantly improve the efficiency of quantum information protocols.
Because state preparation and measurement are relatively straightforward in this approach, Bhattacharya and Raman say it is well-suited for quantum computing. Since the method relies on geometric principles, it naturally protects against certain types of noise, making it more robust than traditional approaches. They add that their technique could even be used to construct an almost universal set of near-deterministic entangling gates for quantum computation with light. “This innovative use of non-Abelian holonomy could shift the way we think about photonic quantum computing,” they say.
By providing a deterministic and scalable entanglement mechanism, Bhattacharya and Raman add that their method opens the door to more efficient and reliable photonic quantum technologies. The next steps will be to validate the approach experimentally and explore practical implementations in quantum communication and computation. Further in the future, it will be necessary to find ways of integrating this approach with other quantum systems, such as matter-based qubits, to enable large-scale quantum networks.
The post New entanglement approach could boost photonic quantum computing appeared first on Physics World.

COLORADO SPRINGS — The commercial geostationary (GEO) satellite market is undergoing rapid transformation. Operators are adapting to shrinking broadcast revenues, increased pressure from low Earth orbit (LEO) constellations and the […]
The post Q&A: Swissto12 CEO Emile de Rijk explains why he thinks the market is shifting his way appeared first on SpaceNews.

Colorado Springs — Within days of each other in late February and early March, the Aerospace Corp. and Spire Global announced similar breakthroughs: demonstrating optical intersatellite links between cubesats. News […]
The post Seeing the light: Cubesats share optical data appeared first on SpaceNews.

It’s 2027. Chinese warships have encircled Taiwan. High above, China’s growing fleet of satellites track and target United States military forces across the Pacific. At the same time, an array […]
The post How to accelerate U.S. space acquisition and outpace threats appeared first on SpaceNews.
Water molecules on the surface of an electrode flip just before they give up electrons to form oxygen – a feat of nanoscale gymnastics that explains why the reaction takes more energy than it theoretically should. After observing this flipping in individual water molecules for the first time, scientists at Northwestern University in the US say that the next step is to find ways of controlling it. Doing so could improve the efficiency of the reaction, making it easier to produce both oxygen and hydrogen fuel from water.
The water splitting process takes place in an electrochemical cell containing water and a metallic electrode. When a voltage is applied to the electrode, the water splits into oxygen and hydrogen via two separate half-reactions.
The problem is that the half-reaction that produces oxygen, known as the oxygen evolution reaction (OER), is difficult and inefficient and takes more energy than predicted by theory. “It should require 1.23 V,” says Franz Geiger, the Northwestern physical chemist who led the new study, “but in reality, it requires more like 1.5 or 1.8 V.” This extra energy cost is one of the reasons why water splitting has not been implemented on a large scale, he explains.
In the new work, Geiger and colleagues wanted to test whether the orientation of the water’s oxygen atoms affects the kinetics of the OER. To do this, they directed an 80-femtosecond pulse of infrared (1034 nm) laser light onto the surface of the electrode, which was in this case made of nickel. They then measured the intensity of the reflected light at half the incident wavelength.
This method, which is known as second harmonic and vibrational sum-frequency generation spectroscopy, revealed that the water molecules’ alignment on the surface of the electrode depended on the applied voltage. By analysing the amplitude and phase of the signal photons as this voltage was cycled, the researchers were able to pin down how the water molecules arranged themselves.
They found that before the voltage was applied, the water molecules were randomly oriented. At a specific applied voltage, however, they began to reorient. “We also detected water dipole flipping just before cleavage and electron transfer,” Geiger adds. “This allowed us to distinguish flipping from subsequent reaction steps.”
The researchers’ explanation for this flipping is that at high pH levels, the surface of the electrode is negatively charged due to the presence of nickel hydroxide groups that have lost their protons. The water molecules therefore align with their most positively charged ends facing the electrode. However, this means that the ends containing the electrons needed for the OER (which reside in the oxygen atoms) are pointing away from the electrode. “We hypothesized that water molecules must flip to align their oxygen atoms with electrochemically active nickel oxo species at high applied potential,” Geiger says.
This idea had not been explored until now, he says, because water absorbs strongly in the infrared range, making it appear opaque at the relevant frequencies. The electrodes typically employed are also too thick for infrared light to pass through. “We overcame these challenges by making the electrode thin enough for near-infrared transmission and by using wavelengths where water’s absorbance is low (the so-called ‘water window’),” he says.
Other challenges for the team included designing a spectrometer that could measure the second harmonic generation amplitude and phase and developing an optical model to extract the number of net-aligned water molecules and their flipping energy. “The full process – from concept to publication – took three years,” Geiger tells Physics World.
The team’s findings, which are detailed in Science Advances, suggest that controlling the orientation of water at the interface with the electrode could improve OER catalyst performance. For example, surfaces engineered to pre-align water molecules might lower the kinetic barriers to water splitting. “The results could also refine electrochemical models by incorporating structural water energetics,” Geiger says. “And beyond the OER, water alignment may also influence other reactions such as the hydrogen evolution reaction and CO₂ reduction to liquid fuels, potentially impacting multiple energy-related technologies.”
The researchers are now exploring alternative electrode materials, including NiFe and multi-element catalysts. Some of the latter can outperform iridium, which has traditionally been the best-performing electrocatalyst, but is very rare (it comes from meteorites) and therefore expensive. “We have also shown in a related publication (in press) that water flipping occurs on an earth-abundant semiconductor, suggesting broader applicability beyond metals,” Geiger reveals.
The post Splitting water takes more energy than theory predicts – and now scientists know why appeared first on Physics World.

The program will distribute unclassified threat data to more than 900 space companies registered through the Space Systems Command's "Front Door" portal
The post Space Force announces ‘Orbital Watch’ program to share intelligence with commercial sector appeared first on SpaceNews.

The company is expanding its small-satellite production space from 22,000 square feet to nearly 42,000 square feet.
The post Boeing’s Millennium Space expands production to meet defense demand appeared first on SpaceNews.

Paris, April 5, 2025 — One year after launching as a unified brand, Novaspace marks a major milestone as the trusted and independent global consulting firm guiding governments, space agencies, […]
The post Novaspace Marks One Year as a Unified Brand — Built on 40+ Years of Space Expertise appeared first on SpaceNews.